The method of identifying a substance based on its spectrum and determining its chemical composition and relative content is called spectral analysis. Its advantages are sensitive and rapid. Historically, many new elements have been discovered through spectral analysis, such as 铷, 铯, 氦, etc.
The analysis principle is that the characteristic spectrum of the element to be tested radiated by the light source is absorbed by the ground state atoms of the element to be tested in the vapor of the sample, and the extent of the emission spectrum is weakened, thereby obtaining the content of the element to be tested in the sample, which is in accordance with Lang. Per-Bill law
A= -lg I/I o= -lgT = KCL
Where I is the transmitted light intensity, I0 is the emitted light intensity, T is the transmittance, and L is the light passing through the atomizer optical path since L is a constant value so A = KC.
The physical principle is:
The atoms of any element are composed of nuclei and electrons moving around the nucleus. The electrons outside the nucleus are layered according to the level of their energy to form different energy levels. Therefore, a nucleus can have multiple energy levels.
The lowest energy level is called the ground state level (E0 = 0), the other energy level is called the excited state level, and the lowest excited state is called the first excited state. Under normal conditions, the atoms are in the ground state, and the extranuclear electrons move in the orbit with the lowest energy.
If a certain external energy, such as light energy, is supplied to the ground state atom, when the external light energy E is exactly equal to the energy level difference E between the ground state and a higher energy level in the ground state atom, the atom will absorb light of this characteristic wavelength. The outer electrons transition from the ground state to the corresponding excited state to produce an atomic absorption spectrum.
After the electron transition to the higher energy level, it is in an excited state, but the excited state electron is unstable. After about 10-8 seconds, the excited state electron will return to the ground state or other lower energy level, and the energy absorbed by the electron transition. Released in the form of light, this process is called atomic emission spectroscopy. It can be seen that the atomic absorption spectroscopy process absorbs radiant energy, while the atomic emission spectroscopy process releases radiant energy.
The analysis principle is that the characteristic spectrum of the element to be tested radiated by the light source is absorbed by the ground state atoms of the element to be tested in the vapor of the sample, and the extent of the emission spectrum is weakened, thereby obtaining the content of the element to be tested in the sample, which is in accordance with Lang. Per-Bill law
A= -lg I/I o= -lgT = KCL
Where I is the transmitted light intensity, I0 is the emitted light intensity, T is the transmittance, and L is the light passing through the atomizer optical path since L is a constant value so A = KC.
The physical principle is:
The atoms of any element are composed of nuclei and electrons moving around the nucleus. The electrons outside the nucleus are layered according to the level of their energy to form different energy levels. Therefore, a nucleus can have multiple energy levels.
The lowest energy level is called the ground state level (E0 = 0), the other energy level is called the excited state level, and the lowest excited state is called the first excited state. Under normal conditions, the atoms are in the ground state, and the extranuclear electrons move in the orbit with the lowest energy.
If a certain external energy, such as light energy, is supplied to the ground state atom, when the external light energy E is exactly equal to the energy level difference E between the ground state and a higher energy level in the ground state atom, the atom will absorb light of this characteristic wavelength. The outer electrons transition from the ground state to the corresponding excited state to produce an atomic absorption spectrum.
After the electron transition to the higher energy level, it is in an excited state, but the excited state electron is unstable. After about 10-8 seconds, the excited state electron will return to the ground state or other lower energy level, and the energy absorbed by the electron transition. Released in the form of light, this process is called atomic emission spectroscopy. It can be seen that the atomic absorption spectroscopy process absorbs radiant energy, while the atomic emission spectroscopy process releases radiant energy.
1KW-6KW Hybrid Inverter (with PWM Charge)

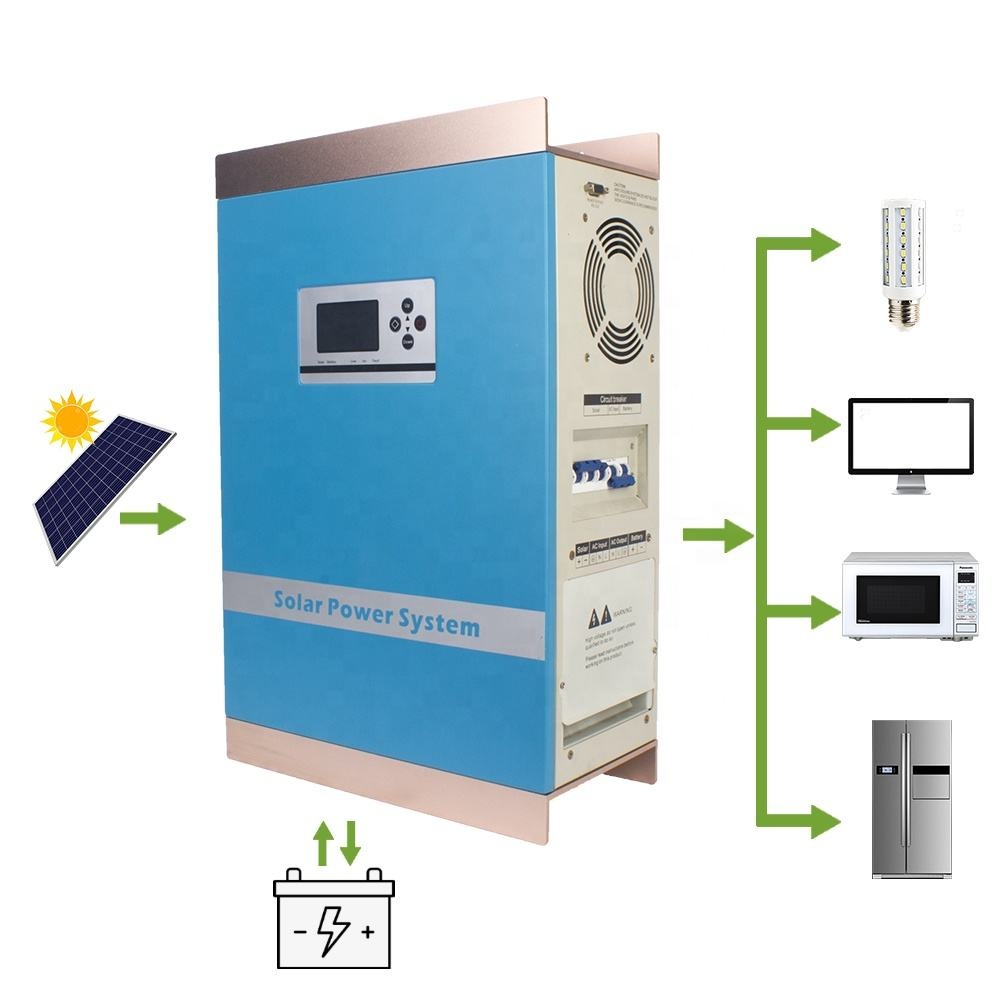
1KW-6KW PWM Hybrid Inverter,Lithium Battery Solar Inverter,AC 220V Solar Hybrid Inverter
suzhou whaylan new energy technology co., ltd , https://www.nbwhaylan.com